Literature Automation Tool
ARS PV is a pharmacovigilance workflow management tool that centralizes the discovery of critical adverse event information in various types of literature, including information found in the Medical Literature Monitoring (MLM).
Artificial Intelligence (AI), where in the system can interpret and analysis the source (source documents) and pick the appropriate content and perform case intake, seriousness assessment, case processing and medical review (to an extent) and case monitoring basis machine learning.
Literature surveillance is an incessant activity for Good pharmacovigilance practices. If this process is technology-aided in conjunction with the automated search hits from global medical literature, the resources can work efficiently in early safety risk detection after reviewing and categorizing literature of interest for safety reporting.
How ARS PV helps you in Literature Surveillance and case creation
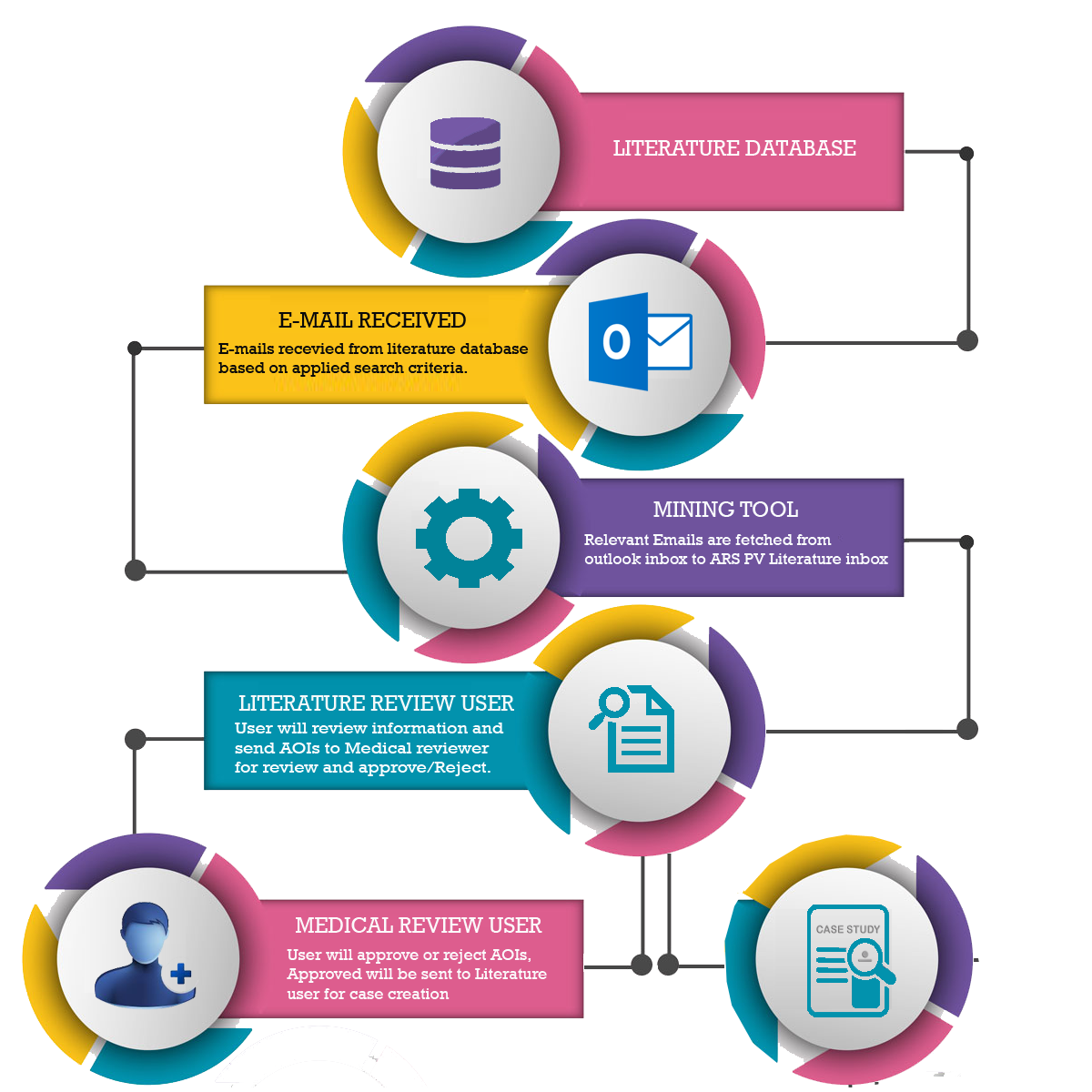
- E-mails notification received on registered E-mail ID from literature publishing platform on registered criteria on literature platform are fetched by the ARS PV literature mining tool, hence you will be able to get your literature search in ARS PV literature mining tool.
- ARS PV literature mining tool identifies the adverse events from a wide platform of literature sources.
- ARS PV enables you review of literature and monitor the Article of interests (AOIs) from a single browser-based interface.
- Hence, enables you to review your literature and move ahead towards case creation with user friendly interface.
- Ensuring compliance with regulatory best practices and within (GxP) regulated environments